How To Draw A Voltaic Cell
Voltaic Cells
- Page ID
- 285
In redox reactions, electrons are transferred from one species to another. If the reaction is spontaneous, energy is released, which tin so be used to practice useful work. To harness this energy, the reaction must be split into 2 separate one-half reactions: the oxidation and reduction reactions. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. In doing so, a Voltaic/ Galvanic Cell is created.
Introduction
When a redox reaction takes place, electrons are transferred from ane species to the other. If the reaction is spontaneous, free energy is released, which can be used to practise piece of work. Consider the reaction of a solid copper (Cu(due south)) in a argent nitrate solution (AgNO3 (s)).
\[2Ag^+_{(aq)} + Cu_{(s)} \leftrightharpoons Cu^{2+}_{(aq)} + 2Ag_{(southward)}\]
The \(AgNO_{3\;(s)}\) dissociates in water to produce \(Ag^+_{(aq)}\) ions and \(NO^-_{iii\;(aq)}\) ions. The NO3 - (aq) ions tin can be ignored since they are spectator ions and do not participate in the reaction. In this reaction, a copper electrode is placed into a solution containing silver ions. The Ag+ (aq) will readily oxidize Cu(south) resulting in Cu2 + (aq), while reducing itself to Ag(southward).
This reaction releases energy. When the copper electrode solid is placed directly into a silvery nitrate solution, nonetheless, the energy is lost as heat and cannot exist used to do work. In order to harness this energy and utilize information technology do useful work, nosotros must divide the reaction into two split one-half reactions; The oxidation and reduction reactions. A wire connects the two reactions and allows electrons to flow from one side to the other. In doing and then, nosotros have created a Voltaic/ Galvanic Cell.
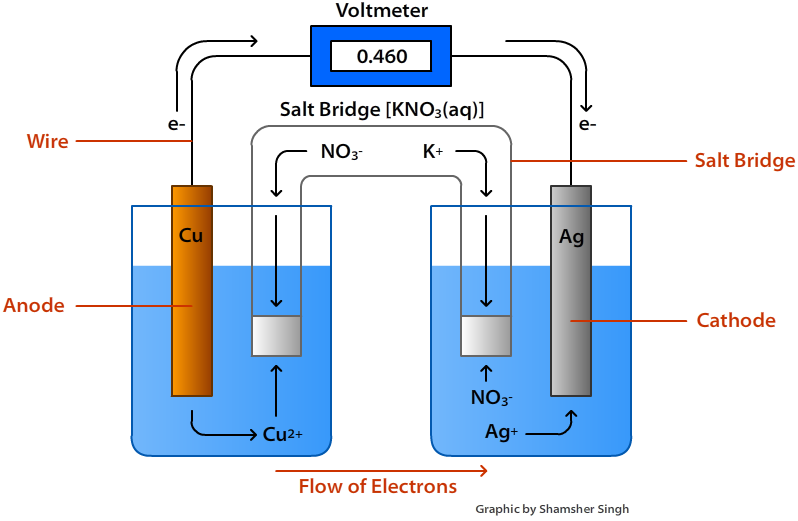
Effigy \(\PageIndex{i}\): Voltaic Cell
A Voltaic Cell (likewise known every bit a Galvanic Cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It consists of two separate one-half-cells. A one-half-cell is composed of an electrode (a strip of metallic, M) inside a solution containing Mdue north+ ions in which M is any arbitrary metal. The two one-half cells are linked together past a wire running from one electrode to the other. A salt bridge besides connects to the half cells. The functions of these parts are discussed below.
Half Cells
Half of the redox reaction occurs at each half prison cell. Therefore, nosotros can say that in each one-half-cell a half-reaction is taking place. When the two halves are linked together with a wire and a salt bridge, an electrochemical cell is created.
Electrodes
An electrode is strip of metal on which the reaction takes place. In a voltaic prison cell, the oxidation and reduction of metals occurs at the electrodes. In that location are ii electrodes in a voltaic cell, one in each half-prison cell. The cathode is where reduction takes identify and oxidation takes identify at the anode.
Through electrochemistry, these reactions are reacting upon metallic surfaces, or electrodes. An oxidation-reduction equilibrium is established between the metallic and the substances in solution. When electrodes are immersed in a solution containing ions of the same metal, it is chosen a half-cell. Electrolytes are ions in solution, normally fluid, that conducts electricity through ionic conduction. Two possible interactions tin can occur betwixt the metal atoms on the electrode and the ion solutions.
- Metallic ion Kdue north + from the solution may collide with the electrode, gaining "n" electrons from information technology, and catechumen to metal atoms. This means that the ions are reduced.
- Metal atom on the surface may lose "n" electrons to the electrode and enter the solution as the ion Thousandnorth + meaning that the metal atoms are oxidized.
When an electrode is oxidized in a solution, it is chosen an anode and when an electrode is reduced in solution. information technology is chosen a cathode.
- Anode: The anode is where the oxidation reaction takes place. In other words, this is where the metallic loses electrons. In the reaction above, the anode is the Cu(due south) since information technology increases in oxidation country from 0 to +two.
- Cathode: The cathode is where the reduction reaction takes place. This is where the metal electrode gains electrons. Referring dorsum to the equation higher up, the cathode is the Ag(s) as it decreases in oxidation state from +1 to 0.
Remembering Oxidation and Reduction
When information technology comes to redox reactions, it is important to sympathize what it ways for a metal to be "oxidized" or "reduced". An easy manner to do this is to remember the phrase "OIL RIG".
OIL = Oxidization is Loss (of eastward-)
RIG = Reduction is Gain (of e-)
In the instance of the example higher up \(Ag^+_{(aq)}\) gains an electron meaning it is reduced. \(Cu_{(s)}\) loses two electrons thus information technology is oxidized.
The salt bridge is a vital component of whatever voltaic cell. It is a tube filled with an electrolyte solution such every bit KNO3(s) or KCl(s). The purpose of the salt span is to continue the solutions electrically neutral and let the free flow of ions from one jail cell to another. Without the common salt bridge, positive and negative charges will build upwardly around the electrodes causing the reaction to terminate.
The purpose of the salt bridge is to keep the solutions electrically neutral and allow the complimentary flow of ions from one jail cell to another.
Flow of Electrons
Electrons e'er flow from the anode to the cathode or from the oxidation one-half jail cell to the reduction one-half cell. In terms of Eastwardocell of the one-half reactions, the electrons will flow from the more negative one-half reaction to the more positive half reaction. A cell diagram is a representation of an electrochemical cell. The effigy below illustrates a cell diagram for the voltaic shown in Figure \(\PageIndex{1}\) higher up.
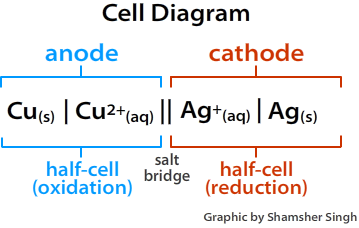
Figure \(\PageIndex{ii}\): Cell Diagram. The figure below illustrates a cell diagram for the voltaic shown in Figure \(\PageIndex{1}\).
When drawing a cell diagram, we follow the following conventions. The anode is ever placed on the left side, and the cathode is placed on the correct side. The salt bridge is represented by double vertical lines (||). The deviation in the stage of an element is represented by a single vertical line (|), while changes in oxidation states are represented by commas (,).
When asked to construct a cell diagram follow these simple instructions. Consider the following reaction:
\[2Ag^+_{(aq)} + Cu_{(southward)} \rightleftharpoons Cu^{2+}_{(aq)} + 2Ag_{(s)}\]
Footstep 1: Write the two half-reactions.
\[Ag^+_{(aq)} + east^- \rightleftharpoons Ag_{(s)}\]
\[Cu_{(s)} \rightleftharpoons Cu^{ii+}_{(aq)} + 2e^-\]
Step 2: Identify the cathode and anode.
\(Cu_{(south)}\) is losing electrons thus being oxidized; oxidation occurs at the anode.
- Anode (where oxidation occurs): \(Cu_{(s)} \rightleftharpoons Cu^{2+}_{(aq)} + 2e^-\)
\(Ag^+\) is gaining electrons thus is being reduced; reduction happens at the cathode.
- Cathode (where reduction occurs): \(Ag^+_{(aq)} + e^- \rightleftharpoons Ag_{(due south)}\)
Step 3: Construct the Jail cell Diagram.
\[Cu_{(southward)} | Cu^{2+}_{(aq)} || Ag^+_{(aq)} | Ag_{(due south)}\]
The anode always goes on the left and cathode on the right. Split changes in phase past | and indicate the the table salt bridge with ||. The lack of concentrations indicates solutions are under standard atmospheric condition (i.due east., 1 1000)
Instance \(\PageIndex{1}\)
Consider the following two reactions:
\[Cu^{2+}_{(aq)} + Ba_{(s)} \rightarrow Cu_{(s)} + Ba^{2+}_{(aq)}\]
\[2Al_{(s)} + 3Sn^{2+}_{(aq)} \rightarrow 2Al^{iii+}_{(aq)} + 3Sn_{(s)}\]
- Split the reaction into half reactions and determine their standard reduction potentials. Indicate which would be the anode and cathode.
- Construct a cell diagram for the following each reactions.
- Determine the \(Eastward^o_{prison cell}\) for the voltaic jail cell formed by each reaction.
Solution
1.a) Batwo+ (aq) → Ba(southward) + 2e- with SRP (for opposite reaction) Eo = -2.92 V (Anode; where oxidation happens)
Cu two+ (aq) + 2e- → Cu(s) with SRP Eo = +0.340 V (Cathode; where reduction happens)
one.b) Al3+ (aq) → Al(southward) + 3e- with SRP (for contrary reaction) Eo = -ane.66 V (Anode; where oxidation happens)
Sn2+ (aq) +2e- → Sn(s) with SRP Eo = -0.137 Five (Cathode; where reduction happens)
2.a) Ba2+ (aq) | Ba(s) || Cu(s) | Cu 2+ (aq)
2.b) Al(s) | Al3+ (aq) || Sntwo+ (aq) | Sn(southward)
3.a) Eo cell = 0.34 - (-2.92) = three.26 V
iii.b) Eo cell = -0.137 - (-1.66) = 1.523 V
Cell Voltage/Jail cell Potential
The readings from the voltmeter give the reaction's jail cell voltage or potential difference betwixt it's two two half-cells. Cell voltage is also known as cell potential or electromotive force (emf) and it is shown as the symbol \(E_{cell}\).
Standard Cell Potential:
\[E^o_{cell} = E^o_{cathode} - Due east^o_{anode}\]
The Eo values are tabulated with all solutes at i M and all gases at i atm. These values are called standard reduction potentials. Each half-reaction has a dissimilar reduction potential, the difference of two reduction potentials gives the voltage of the electrochemical cell. If Eojail cell is positive the reaction is spontaneous and it is a voltaic cell. If the Eastojail cell is negative, the reaction is non-spontaneous and it is referred to equally an electrolytic cell.
References
- Brady, James E., Holum, John R. "Chemistry: The Study of Matter and Its Changes", John Wiley & Sons Inc 1993
- Brown, Theodore L., LeMay, H. Eugene Jr. "Chemistry: The Central Science" Third Edition, Prentice-Hall, Inc. Englewood Cliffs, N.J. 07632 1985
- Brown, Theodore L., LeMay, H. Eugene Jr., Bursten, Bruce E. "Chemistry: The Central Science" Fifth Edition, Prentice-Hall, Inc. Englewood Cliffs, Due north.J. 07632 1991
- Gesser, Hyman D. " Descriptive Principles of Chemistry", C.Five. Mosby Company 1974
- Harwood, William, Herring, Geoffrey, Madura, Jeffry, and Petrucci, Ralph, General Chemical science: Principles and Mod Applications, Ninth Edition, Upper Saddle River,New Bailiwick of jersey, Pearson Prentice Hall, 2007.
- Petrucci, Ralph H. Genereal Chemical science: Principles and Modern Applications ninth Ed. New Jersey: Pearson Education Inc. 2007.
- Vassos Basil H. Electroanaytical Chemistry. New York: Wiley-Interscience Publication. 1983.
- Zumdahl, Steven S. Chemistry 7th Ed. New York: Houghton Mifflin Company. 2007.
Contributors and Attributions
- Shamsher Singh, Deborah Gho
Source: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Voltaic_Cells
Posted by: bateswilty1948.blogspot.com


0 Response to "How To Draw A Voltaic Cell"
Post a Comment